Novel bioinspired polymeric material
Concepts and applications for functionalized contact lenses and keratoprostheses
The development of biofunctionalized materials represents a paradigm shift in contact lens technology. By mimicking natural biological structures such as mucins through synthetic (glyco)polymers, researchers are creating advanced materials with tailored properties for improved wearing comfort, therapeutic drug delivery, enhanced biocompatibility, and diagnostic applications. These biomimetic approaches significantly reduce friction and irritation while enabling sustained drug release and real-time biosensing. This approach extends beyond contact lenses to keratoprostheses, demonstrating the broad potential of bio-inspired polymer design in ophthalmology.
Biofunctional materials are defined as materials that are either derived from biomass or interact specifically with biological systems1. In the context of ophthalmic applications, biofunctionalization refers to the targeted modification of material surfaces to achieve specific biological responses, such as improved biocompatibility, controlled cell adhesion, antimicrobial activity, or targeted drug delivery2. The key advantage of biofunctional materials lies in their ability to bridge the gap between synthetic materials and living tissue, enabling seamless integration and enhanced performance in physiological environments.
The concept of functional bioconjugation encompasses several strategies, including the conjugation of proteins with biofunctionalized polymers to enhance protein stability, enable targeted delivery, facilitate cell uptake, and preserve enzymatic activity. Additionally, surface coatings can be designed to prevent unwanted adhesion, promote controlled cell adhesion like an artificial extracellular matrix (ECM), enable sustained drug release, and improve overall biocompatibility3.
Mucins: Nature’s blueprint for lubrication and protection
Mucins are high-molecular-weight glycoproteins that form the structural basis of the mucus layer covering epithelial surfaces, including the ocular surface. These natural biopolymers consist of a protein backbone densely decorated with oligosaccharide side chains, creating a highly hydrated, gel-like structure4. The glycosylated regions of mucins, such as those found in MUC1, provide exceptional lubrication, protect underlying tissues from mechanical stress, and serve as a barrier against pathogens5.
The unique properties of mucins have inspired researchers to develop synthetic analogs that can replicate these functions. In the tear film, mucins play a critical role in maintaining ocular surface health by stabilizing the tear layer, reducing friction during blinking, and preventing desiccation of the corneal epithelium6. Disruption of the mucin layer, whether due to contact lens wear or ocular disorders, can lead to discomfort, inflammation, and compromised visual function. Therefore, artificial glycopolymers that mimic natural mucins offer a promising strategy for enhancing lubrication and protecting the ocular surface in contact lens applications7 as well as in developing new functionalized biomaterials for specific interactions with target (regiospecific) cells (figure 1).
Synthetic glycopolymers: Mimicking mucins with precision
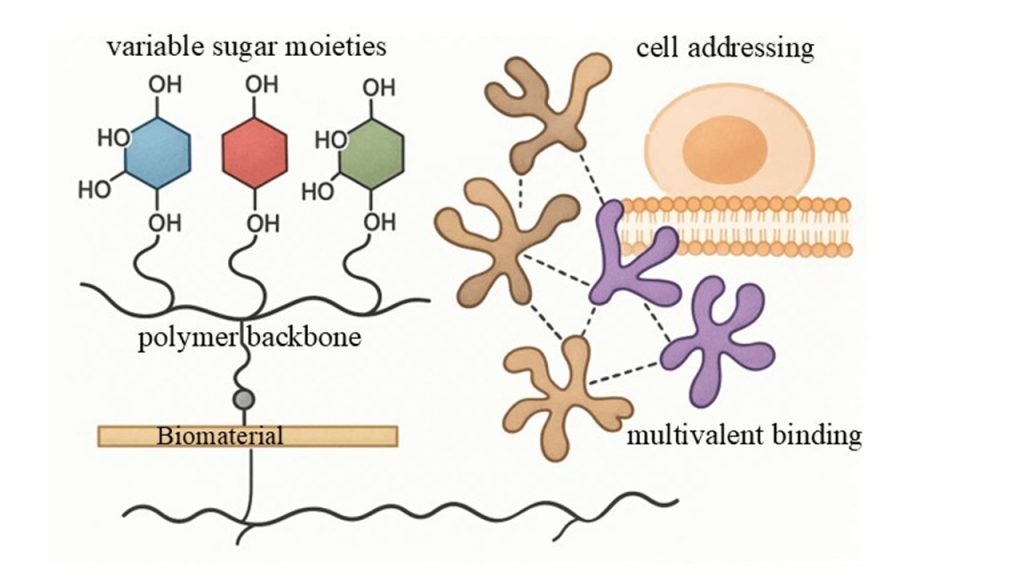
Glycopolymers are synthetic polymers bearing pendant carbohydrate moieties, designed to replicate the structure and function of natural glycoconjugates8. Unlike native mucins, which exhibit complex and heterogeneous structures, synthetic glycopolymers can be precisely engineered using controlled polymerization techniques such as reversible addition-fragmentation chain transfer (RAFT) polymerization9. This allows for fine-tuning of molecular weight, polymer architecture, carbohydrate density, and charge distribution.
The design of artificial mucin mimics typically involves a polymer backbone functionalized with densely packed short glycosides. The polymer backbone serves as the structural scaffold and provides the means for attachment to the target surface, while the pendant sugar residues mediate specific interactions with lectins, proteins, and cells10. By varying the polymer backbone (neutral or charged) and the type of sugar moieties (e.g., lactose, galactose, N-acetylgalactosamine), researchers can tailor the properties of glycopolymers to meet specific biological requirements11.
Recent studies have demonstrated the synthesis of glycopolymers with both positively charged (e.g., polyethyleneimine, PEI) and negatively charged (e.g., sulfated lactose) components. These charged glycopolymers can be assembled into multilayer thin films using layer-by-layer (LbL) deposition techniques, creating stable coatings on various substrates, including contact lenses12. Importantly, glycomodification of the polymer backbone significantly enhances coating stability compared to non-glycosylated polymers, highlighting the crucial role of sugar moieties in promoting robust surface functionalization12.
Applications in contact lens technology
Enhanced biocompatibility and lubrication
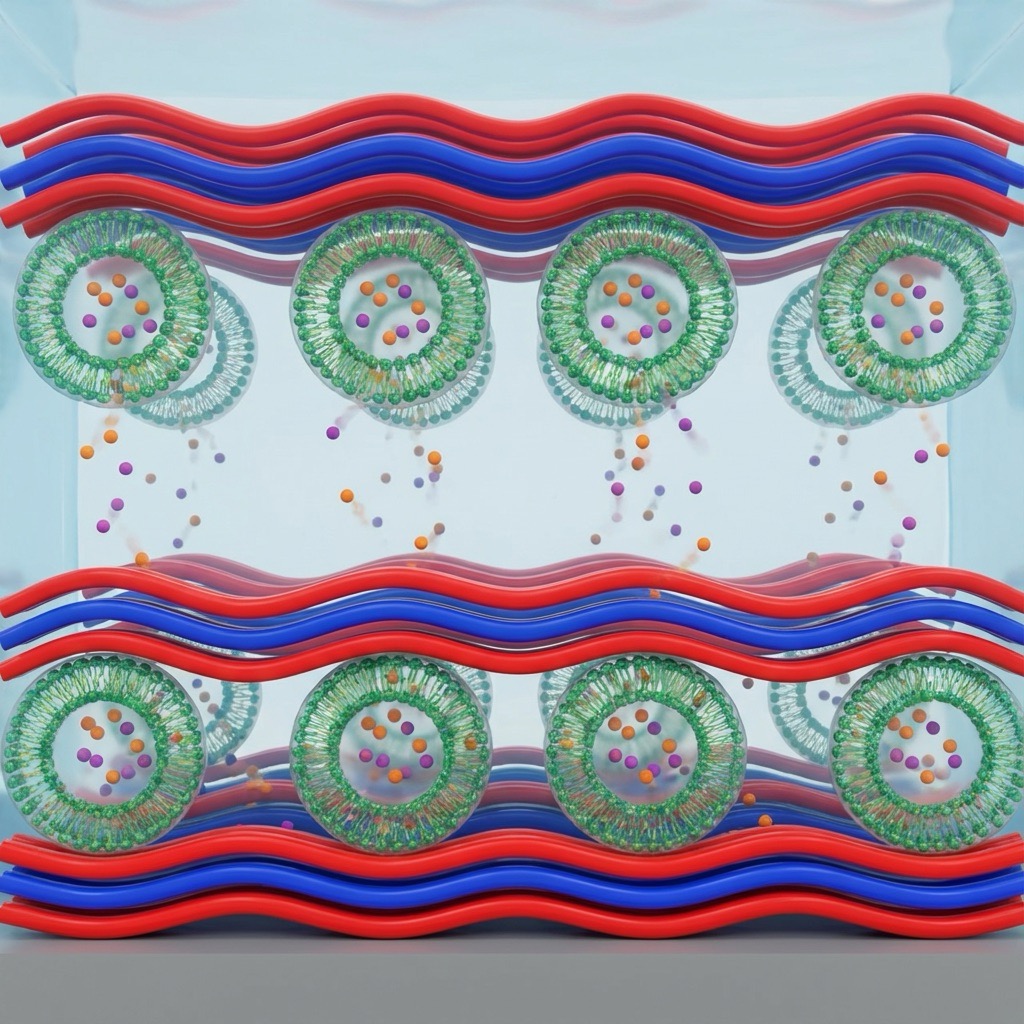
The application of mucin-mimicking glycopolymers to contact lens surfaces has shown promising results in improving biocompatibility and reducing friction. Biocompatibility analyses of artificial mucin-like glycopolymers have confirmed that selected polymers exhibit no cytotoxic effects on human corneal epithelial cells, making them suitable as semi-synthetic materials for ocular surface lubrication and protection7. Coating contact lenses with alternating layers of positively and negatively charged glycopolymers results in a stable, hydrophilic surface that mimics the natural tear film, thereby enhancing wearing comfort and reducing the risk of ocular irritation (Fig. 2)12,13.
Therapeutic contact lenses for drug delivery
One of the most exciting applications of biofunctionalized contact lenses is in the field of ocular drug delivery. Conventional eye drops suffer from poor bioavailability, with less than 5% of the administered drug reaching the target tissue, while more than 30% can cause ocular irritation due to preservatives and high concentrations14. Therapeutic contact lenses offer a promising alternative by providing sustained and controlled drug-release directly to the ocular surface15, 16.
A particularly innovative approach involves the integration of drug-loaded liposomes into glycopolymer-coated contact lenses. Liposomes serve as reservoirs for active pharmaceutical ingredients, protecting them from degradation and enabling prolonged release17. The glycopolymer coating acts as a mucin-mimetic layer that anchors the liposomes to the lens surface through specific carbohydrate-lectin interactions, preventing premature drug leakage while maintaining biocompatibility12. This biofunctional liposome-polymer system addresses the dual challenge of low drug uptake and high irritation rates associated with conventional eye drops.
Commercial examples, such as the Hyper-CL lens developed by EyeYon Medical (figure 3), demonstrate the clinical potential of drug-eluting contact lenses. The unique design of the Hyper-CL® lens creates a reservoir that captures and holds therapeutic eye drops against the cornea, extending contact time and enhancing drug efficacy. The lens can remain in the eye for up to seven days before cleaning, then for another seven days, significantly improving patient compliance and therapeutic outcomes18.
Diagnostic and theranostic applications
Beyond drug delivery, glycopolymers are emerging as key enablers of diagnostic and theranostic (combined diagnostic and therapeutic) contact lens systems. The ability of glycopolymers to undergo specific biorecognition through carbohydrate-lectin interactions makes them ideal candidates for biosensing applications19. For instance, glycopolymer-based sensor arrays can differentiate lectins with similar carbohydrate recognition preferences, enabling the detection of disease-specific biomarkers in tear fluid20.
Smart contact lenses equipped with glycopolymer-functionalized biosensors have been developed for continuous monitoring of glucose levels in diabetic patients, as well as for detecting inflammatory markers such as matrix metalloproteinase-9 (MMP-9) in dry eye disease21, 22. These systems leverage the high precision, selectivity, and sensitivity of glycopolymer-lectin interactions to provide real-time, non-invasive monitoring, thereby improving patient quality of life and enabling personalized medicine approaches23.

From contact lenses to keratoprostheses
Biofunctionalization of optics and haptics
The principles of biofunctionalization extend beyond contact lenses to artificial corneas, or keratoprostheses. A keratoprosthesis must fulfill multiple, sometimes conflicting, requirements: the optical component must remain transparent to ensure vision, while the haptic component (the “skirt” that anchors the device) must promote tissue integration and cell ingrowth24, 25. Achieving all of these properties in a single base material is a significant challenge, necessitating differential biofunctionalization of the optic and haptic regions.
The Miro Cornea UR keratoprosthesis, developed by Fraunhofer IAP and project partners within the EU-international project „Miro“, exemplifies this approach. The device consists of a very hydrophobic copolymer core. The anterior optic is modified with a hydrophilic interpenetrating polymer network to enhance wettability and optical clarity, while the haptic is functionalized with fibronectin-like peptides (FnLeP) to promote rapid cell adhesion and tissue ingrowth25. This dual functionalization strategy ensures that the implant interacts appropriately with different tissue types and fulfills distinct functional requirements.
The coating process for the Miro® keratoprosthesis involves multiple steps. The anterior optic undergoes plasma treatment followed by photo-initiated polymerization of hydrophilic monomers, resulting in a hydrophilically coated surface. The haptic regions are coated using layer-by-layer deposition of polyelectrolytes, such as alternating layers of chitosan and heparin, or peptide layers, to create a bioactive interface that facilitates tissue integration 26. Clinical studies have demonstrated successful implantation in ultima ratio patients, with restoration of vision and good long-term biocompatibility25.
Another example of biofunctionalization was the Artcornea®. This keratoprosthesis, made of a hydrophilic polymer composition, was selectively modified on the haptic („skirt“) to promote a stable ingrowth and to prevent implant rejection and inflammatory side effects. Key features are:
- Implantable and biocompatible: Made from a water-absorbing polymer, the implant is designed to be easily integrated into the eye without causing immune rejection.
- Encourages tissue growth: The haptic of the implant is chemically treated to encourage the patient’s own cells to grow and bind, anchoring the prosthesis securely in the eye.
- Improved optics: The design aims to improve the light-gathering area of the cornea, which can lead to better vision.
- Suitable for various conditions: It is intended for patients who cannot receive a donor cornea or who are on a waiting list for one. It can also be used in cases where the cornea is destroyed by inflammation, accidents, chemical burns, or corrosion, though other versions have been specifically developed for this “first aid” application.
- Successful preclinical testing: The artificial cornea has undergone successful laboratory and animal testing.
Outlook and future perspectives: The importance of glycopolymers and advanced biocompatibility testing
Glycopolymers represent an excellent tool for tailoring material properties to specific biological requirements, whether for different cell systems, organs, or functions. Their versatility and tunability make them particularly well-suited for applications in contact lenses, therapeutic drug delivery systems, and keratoprostheses. Moreover, glycopolymers hold high potential in the research and development of novel diagnostic systems with enhanced precision, selectivity, sensitivity, user-friendliness, and improved quality of life for patients23.
However, as biofunctional materials become increasingly sophisticated, so too must the methods used to assess their biocompatibility. Traditional cytotoxicity assays, which measure cell viability in the presence of a material, provide only a limited view of material-tissue interactions. A material may pass standard cytotoxicity tests, appearing non-toxic, yet still elicit subtle but significant biological responses that could affect long-term performance and safety27.
Advanced biocompatibility testing should therefore include the simultaneous determination of various cytokines, chemokines, and growth factors released by region-specific primary cells in contact with the material or active ingredient. This approach enables a more realistic prediction of how materials and drugs will interact with living organisms over extended periods27. For example, the release of pro-inflammatory cytokines such as interleukin-1β (IL-1β) or interleukin-6 (IL-6), or growth factors such as platelet-derived growth factor-BB (PDGF-BB), can indicate the potential for inflammation or immune responses, even in the absence of overt cytotoxicity27. By incorporating comprehensive cytokine and chemokine profiling into biocompatibility assessments, researchers can gain deeper insights into the long-term behavior of biofunctional materials and make more informed decisions during the development process. This is particularly critical for ophthalmic applications, where materials are in prolonged contact with sensitive ocular tissues and where even mild inflammatory responses can compromise visual outcomes and patient comfort. In conclusion, the integration of bioinspired polymer design, advanced functionalization strategies, and rigorous biocompatibility testing is paving the way for the next generation of contact lenses, therapeutic delivery systems, and keratoprostheses. Glycopolymers, with their ability to mimic natural biological structures and mediate specific molecular interactions, stand at the forefront of this exciting frontier in ophthalmology and biomaterials science.
References: Available on request from the editorial office.







